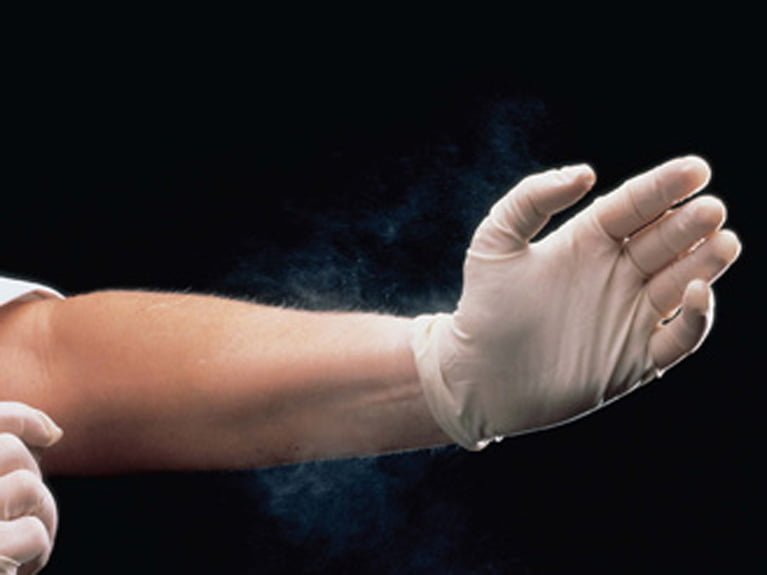
As far as nurses, doctors and medical staff everywhere are concerned, it’s been a long time coming. And today, the Food and Drug Administration have finally proposed to ban the use of powdered gloves.
The proposal is aimed at protecting clinicians and patients by banning nearly all powdered gloves. This includes surgical gloves and patient examination gloves. However, the ban does not apply to powdered radiographic protection gloves.
Clinicians have long been aware of health problems experienced by client and healthcare staff. These include respiratory allergic reactions, as well as post-operative reactions such as adhesions, wound inflammation and granulomas. As a result, many health care facilities already use non-powdered gloves.
Currently, it’s estimated that 93 per cent of clinicians already use powder-free gloves. In fact, large health care industries such as Johns Hopkins Medicine already place severe restrictions on their use.
Professional groups including the American Nurses’ Association and the American College of Surgeons have also taken a stance against powdered gloves.
Adverse health effects of powdered gloves known since the 90s
The FDA first acknowledged the issues with powdered gloves in 1997. It decided not to issue a ban on their use due to the risk of a market shortage leading to inferior products and increased cost. However, along with professional medical groups, there was a growing public consensus that powdered gloves should be avoided, and the FDA received several citizen petitions calling for the ban.
Today, after making their announcement, the FDA says a ban on powdered gloves will cause no significant product shortage or economic increase. Also, due to the current wide use of non-powdered gloves, the proposed ban should have minimal affect on clinical practice.
The FDA will be accepting comments on the proposal for the next 90 days. For more information, or to submit a comment, visit the FDA website.